Phosphonates (or phosphonic acids) are a broad family of organic molecules based on phosphorus (chemical symbol P), carbon (C), oxygen (O) and hydrogen (H).
A variety of phosphonates (including many amino phosphonates) occur naturally and in many different types of organisms. The metabolic functions of phosphonates in organisms include cell signalling, metabolism of cell membrane molecules, and the biological synthesis of natural antibiotics. Some bacteria, yeast and fungi can break down phosphonates and use them as a source of food and/or phosphorus.
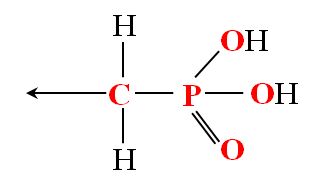
Phosphonates include the chemical group: ‑CH2-PO3H2:
Phosphonic acids |
Phosphonate salts (where M is a metal ion, e.g. sodium) |
 |
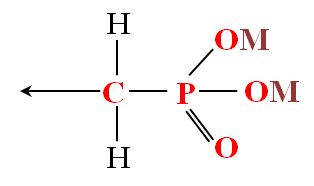 |
Chemical formula of phophonic acids Chemical formula of phophonate salts
(where M is a metal atom such as sodium).
The –CH2-PO3 group confers unique physical and chemical properties on the phosphonates molecules. Because of these properties, phosphonates exhibit:
- High solubility in water
- Strong adsorption on various mineral surfaces
- Ability to sequester (chelate) metal ions
- Inhibition or modification of water hardness deposits
- Resistance to corrosion or oxidation
- Stability under harsh conditions such as acidity, alkalinity or low/high temperatures
- Compatibility with other chemicals and components in formulations.